Introduction
Quality assurance and quality control are vital components of any successful production process, ensuring that products meet established standards and customer expectations. Among the tools used in these processes, AQL samples play a crucial role in determining the acceptability of a batch based on predefined thresholds. Understanding how to effectively implement AQL sampling can significantly influence the overall quality inspection outcomes and enhance the reliability of quality control companies.
Understanding AQL Samples in Quality Control
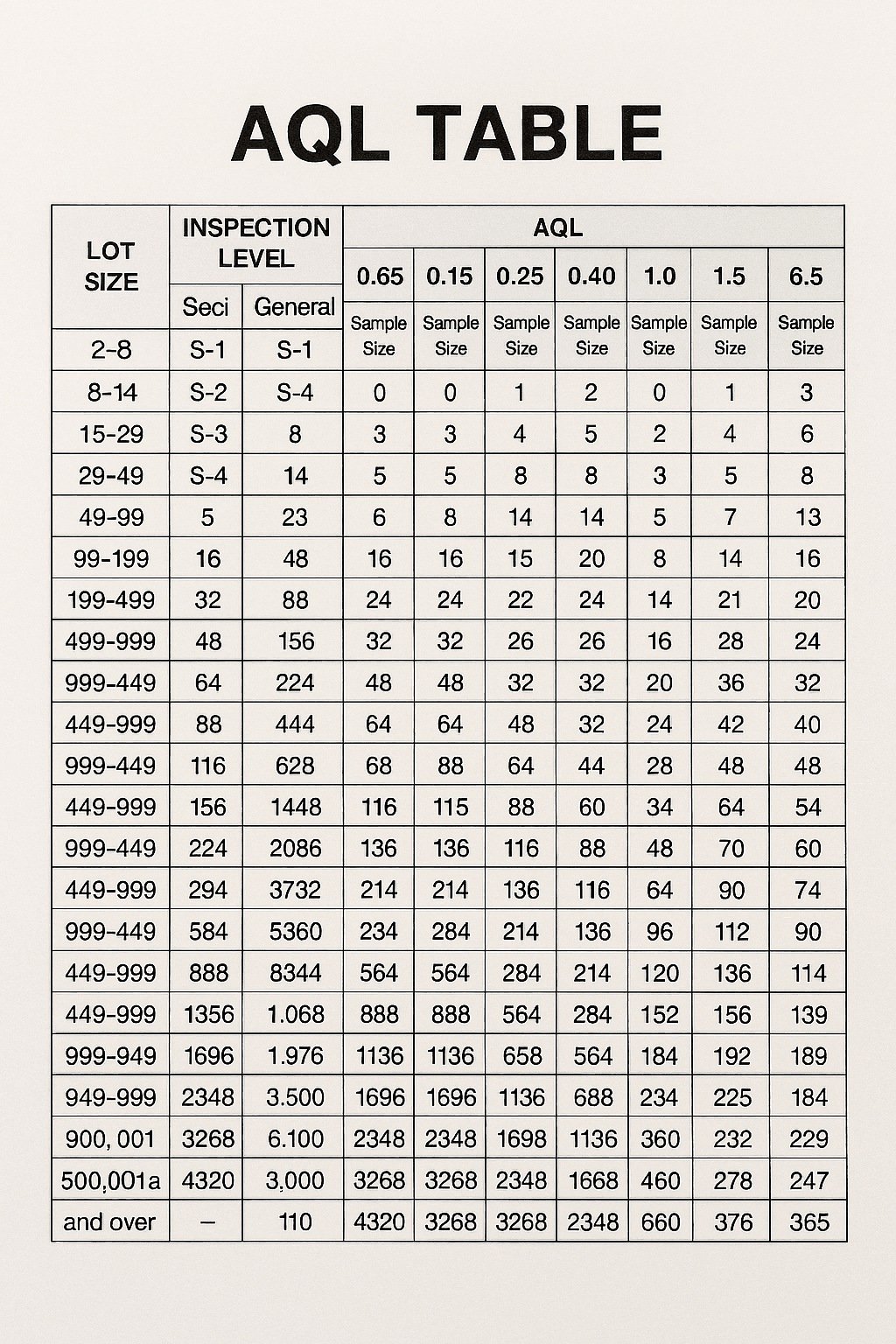
AQL, or Acceptable Quality Level, is a statistical measurement used to determine the maximum number of defective items considered acceptable in a sample batch during quality control inspections. By clearly defining quality through AQL samples, businesses can objectively assess whether their products meet ISO standards and industry benchmarks. Recognizing the importance of AQL samples helps organizations maintain high-quality outputs while minimizing waste and inefficiencies.
Importance of Avoiding Common Mistakes
Mistakes in interpreting or applying AQL levels can lead to serious consequences, including increased costs and diminished customer satisfaction. It’s essential for companies to avoid common pitfalls associated with QC quality control processes by understanding their impact on overall product integrity. By proactively addressing these mistakes, organizations can not only improve their quality assurance practices but also foster trust with clients through consistent delivery of high-quality products.
Overview of Quality Assurance Best Practices
Implementing best practices in quality assurance involves more than just adhering to ISO standards; it requires an ongoing commitment to refining processes and enhancing staff training on critical topics like aql samples and statistical methods. Companies should prioritize clear documentation and robust training programs that empower employees with knowledge about effective sampling strategies for better outcomes in quality inspection. By embracing these best practices, organizations can elevate their QC efforts and ensure long-term success in delivering exceptional products.
Defining Quality Standards

Defining quality is the cornerstone of effective quality assurance and quality control processes. It establishes a clear benchmark against which products and services can be measured, ensuring that they meet customer expectations and regulatory requirements. In the realm of AQL samples, defining quality involves specifying acceptable limits for defects, which directly influences the effectiveness of quality inspection.
What Does It Mean to Define Quality
To define quality means to establish specific criteria that products or services must meet to be considered acceptable. This involves a comprehensive understanding of customer needs and industry standards, including various ISO standards that dictate best practices in quality management. By clearly defining what constitutes quality, organizations can implement robust QC quality control measures that enhance overall performance and reliability.
Quality is not a one-size-fits-all concept; it can vary significantly across industries and sectors. For instance, in manufacturing, it may focus on product durability and functionality, while in service-oriented businesses, it could emphasize customer satisfaction and responsiveness. Thus, defining quality requires an adaptable approach that aligns with both organizational goals and consumer expectations.
Different ISO Standards Explained
ISO standards are internationally recognized benchmarks that guide organizations in establishing effective management systems for various aspects of their operations, including quality assurance. The most relevant standard concerning AQL samples is ISO 9001, which provides a framework for ensuring consistent product or service delivery through continuous improvement processes. Other standards like ISO 13485 target specific industries such as medical devices, emphasizing compliance with stringent regulatory requirements.
Understanding these ISO standards is crucial for companies aiming to enhance their QC quality control measures while meeting global market demands. Compliance not only improves operational efficiency but also builds trust among customers who seek reliable products backed by established safety protocols. Therefore, aligning internal processes with these standards can significantly elevate an organization's reputation in the marketplace.
Moreover, adhering to ISO standards enhances collaboration between different stakeholders involved in the production process— suppliers, manufacturers, and consumers alike—ensuring everyone shares a common understanding of what defines quality within their respective contexts. This collective effort ultimately leads to more effective use of AQL samples during inspections as all parties are aligned on expectations.
The Role of Quality Control Companies
Quality control companies play a vital role in helping organizations define and maintain their quality standards through rigorous testing protocols like AQL sampling methods. These specialized firms provide expertise in areas such as product inspection and compliance audits while offering insights into best practices in QC quality control procedures tailored to specific industries or markets. By leveraging their knowledge base and experience with various ISO standards, these companies help businesses navigate complex regulatory landscapes effectively.
Furthermore, partnering with reputable QC companies allows organizations access to advanced technology tools designed for precise monitoring throughout production cycles—enhancing overall efficiency while minimizing errors during inspections. Their involvement ensures that businesses remain proactive rather than reactive when addressing potential defects or non-conformance issues related to defined quality parameters.
In summary, engaging with professional QC companies not only bolsters an organization’s commitment to maintaining high-quality benchmarks but also fosters continuous improvement efforts essential for long-term success within competitive marketplaces where consumer expectations continuously evolve.
Misinterpretation of AQL Levels
Misinterpretation of AQL (Acceptable Quality Level) levels can lead to significant pitfalls in quality assurance processes. Many quality control companies struggle with this concept, often leading to inconsistencies in quality inspection outcomes. Understanding how to define quality through proper AQL sampling is crucial for maintaining standards and ensuring customer satisfaction.
Common Errors in AQL Sampling
One of the most prevalent errors in AQL sampling is the misunderstanding of the sample size required for inspection. Quality control professionals may select a sample that is either too small or not representative, skewing results and leading to poor decision-making about batch quality. Additionally, failing to differentiate between consumer and producer risks can result in an inappropriate acceptance level, undermining the entire quality assurance process.
Another common mistake occurs when practitioners confuse AQL levels with 100% inspection standards. While some may believe that a higher AQL means better quality, it actually signifies a higher tolerance for defects, which can be misleading during inspections. These errors not only affect the immediate outcomes but can also have long-term repercussions on a company’s reputation and customer trust.
Lastly, many overlook the importance of documentation related to AQL sampling procedures. Without clear records detailing how samples were chosen and tested according to ISO standards, it becomes difficult to replicate successful practices or identify areas needing improvement within QC processes. This lack of clarity can lead to repeated mistakes across batches, further complicating efforts toward achieving consistent quality assurance.
How AQL Levels Impact Quality Inspection
AQL levels play a pivotal role in determining how effectively products are evaluated during quality inspections. By establishing acceptable limits for defects within sampled items, businesses can balance cost-effectiveness with product integrity—ensuring that they don’t reject good products while still maintaining high-quality standards. The choice of an appropriate AQL level directly impacts how many items are inspected and what criteria are used for acceptance or rejection.
Moreover, understanding different ISO standards related to AQL helps guide organizations toward best practices in their QC processes. Companies that properly apply these standards tend to see fewer discrepancies between expected and actual product performance during inspections—leading ultimately to enhanced customer satisfaction and loyalty. Thus, having a solid grasp on how these levels function is essential for effective quality control management.
Finally, misjudging these levels can result in costly recalls or returns if defective products reach customers unnoticed due to lax inspection protocols based on misunderstood tolerances. This highlights the importance of rigorous training on interpreting AQL levels correctly within teams responsible for implementing QC measures across production lines—ensuring everyone involved understands their impact on overall product success.
Real-World Examples of AQL Misinterpretation
Consider a scenario where a toy manufacturer sets an overly lenient AQL level without thoroughly understanding its implications—perhaps accepting up to 5% defects based on misread guidelines from ISO standards related specifically to toys designed for children under three years old! This could lead not only to dangerous products reaching store shelves but also potential legal ramifications if safety regulations are violated due simply misinterpreting acceptable defect rates within those specific contexts.
In another instance, an electronics company might fail by confusing their internal definitions around what constitutes acceptable versus unacceptable defects when applying their chosen aql samples during inspections—resulting in faulty gadgets being shipped out en masse despite numerous complaints from customers regarding functionality issues post-purchase! Such oversights emphasize why clear communication about defined thresholds must exist throughout all departments involved—from development through final QC checks before distribution occurs.
Lastly, let’s not forget about food production companies who sometimes misapply statistical methods when determining appropriate sample sizes relative towards ensuring safe consumption by consumers; failing at this stage could mean serious health risks associated with contaminated batches slipping through unnoticed due solely misreading established parameters set forth by relevant iso standard regulations governing food safety checks! It’s crucial that organizations recognize these real-world examples as cautionary tales highlighting just how critical accurately interpreting those pesky yet vital numbers truly is!
Inconsistent Quality Control Procedures
In the world of quality assurance, consistency is the golden rule. When it comes to QC quality control, having a standard approach ensures that every product meets the defined quality benchmarks without fail. Inconsistent procedures can lead to variability in results, which ultimately jeopardizes the integrity of quality inspection and customer satisfaction.
Importance of Consistency in QC Quality Control
Consistency in QC quality control is crucial for maintaining trust and reliability in any production process. When companies fail to define quality standards consistently, they risk producing products that vary widely in quality, leading to customer complaints and potential returns. By adhering to a uniform set of procedures, organizations can ensure that their products not only meet but exceed ISO standards set forth by industry regulators.
Establishing a Reliable Quality Control Process
Establishing a reliable quality control process requires careful planning and execution. First, companies should assess their existing protocols and identify areas where inconsistencies may arise; this often involves collaborating with reputable quality control companies for insights into best practices. Once weaknesses are identified, organizations can implement standardized procedures that align with their goals for AQL samples while ensuring compliance with relevant ISO standards.
Tips for Maintaining Quality Across Batches
Maintaining consistent quality across batches is no small feat; however, there are several strategies that can help streamline this effort. Regular training sessions for staff on QC fundamentals will keep everyone on the same page regarding expectations and processes related to AQL samples and overall quality assurance practices. Additionally, utilizing automated systems for documentation can enhance accuracy while minimizing human error—ensuring that each batch adheres strictly to defined standards.
Ignoring Statistical Sampling Techniques

In the realm of quality assurance, overlooking statistical sampling techniques can lead to significant pitfalls. AQL samples are designed to provide a representative snapshot of a larger batch, but without proper statistical methods, these samples may misguide quality control efforts. Understanding and applying statistical principles is crucial for effective quality inspection and ensuring compliance with ISO standards.
The Importance of Statistical Methods in AQL Samples
Statistical methods play an essential role in interpreting AQL samples accurately. They help define quality by providing a framework for evaluating the acceptability of products based on sample data rather than subjective judgment. Quality control companies rely on these techniques to minimize risks and ensure that their QC quality control processes yield reliable results, ultimately leading to higher customer satisfaction.
Moreover, employing statistical sampling allows businesses to identify trends and anomalies within their production processes more effectively. By analyzing data from AQL samples, companies can pinpoint areas needing improvement and take corrective action before issues escalate. This proactive approach not only enhances product quality but also aligns with various ISO standards that emphasize continuous improvement in manufacturing practices.
How to Apply Statistical Techniques Effectively
To apply statistical techniques effectively in your QC quality control procedures, start by selecting the appropriate sampling method based on your specific needs. Common methods include random sampling, stratified sampling, and systematic sampling—each serving different purposes depending on product characteristics and production volume. Once you’ve chosen a method, it’s vital to establish clear criteria for acceptance or rejection based on predefined AQL levels.
Additionally, using software tools can streamline the analysis process and reduce human error when interpreting sample data. These tools can automate calculations related to defect rates or confidence intervals, allowing teams to focus on making informed decisions rather than getting bogged down in numbers. Regular training sessions should also be conducted for staff involved in quality inspection so they remain adept at using these statistical techniques effectively.
Finally, documenting your procedures is crucial for maintaining consistency across batches while adhering to ISO standards. This ensures that everyone involved understands how statistical methods integrate into the overall quality assurance framework while also enabling future audits or evaluations by external parties like quality control companies.
Case Studies on Successful Sampling Strategies
Numerous organizations have successfully implemented robust statistical sampling strategies that enhanced their overall quality assurance efforts through effective use of AQL samples. For example, a leading electronics manufacturer adopted stratified random sampling during their production process which resulted in a 30% reduction in defect rates over six months. By segmenting their products into distinct categories based on complexity and risk factors before conducting inspections, they were able to allocate resources more efficiently while still meeting stringent ISO standards.
Another case study involves a textile company that utilized systematic sampling techniques during fabric inspections—taking samples at regular intervals throughout the production line instead of relying solely on end-of-line checks. This approach allowed them greater insight into potential defects occurring earlier in the process—ultimately decreasing returns due to product flaws by 25%. Their commitment to integrating sound statistical methods into their QC processes not only improved product consistency but also reinforced customer trust.
These case studies exemplify how embracing statistical methods within your quality control framework can yield tangible benefits—elevating both product excellence and operational efficiency while adhering closely to industry best practices for defining quality through AQL samples.
Lack of Clear Documentation

In the realm of quality assurance, clear documentation is not just a luxury—it's a necessity. When it comes to quality control, particularly with AQL samples, a well-documented process ensures that everyone involved understands the standards and procedures that define quality. Without proper documentation, even the best QC quality control measures can fall apart, leading to inconsistencies and potential failures in product quality.
Why Documentation Matters in Quality Assurance
Documentation serves as the backbone of any effective quality assurance program. It provides a roadmap for how to conduct quality inspections and adhere to ISO standards while ensuring compliance with industry regulations. Furthermore, clear documentation helps prevent misinterpretation of AQL levels by establishing a reference point for all stakeholders involved in the QC process.
When teams are equipped with thorough records detailing each step of the QC process, they can more effectively track performance over time and identify areas for improvement. This is especially crucial when working with various quality control companies that may have differing operational standards; consistent documentation fosters alignment across teams. Ultimately, having robust records enhances accountability and builds trust among all parties involved.
Best Practices for Documenting QC Processes
To ensure effective documentation in your QC processes, consider adopting several best practices that promote clarity and consistency. First, outline standard operating procedures (SOPs) for each aspect of your quality control processes—this includes everything from sampling methods to inspection criteria related to AQL samples. By defining these steps clearly, you create a reliable framework that guides your team through every stage of production.
Next, establish a centralized document management system where all relevant records are easily accessible to team members involved in quality assurance tasks. This system should include templates for reports on inspections performed under various ISO standards so that data can be captured uniformly across different projects or batches. Regularly updating this information will help maintain accuracy and relevance as processes evolve over time.
Lastly, encourage feedback from your team about the clarity and usability of documentation materials; this will help refine your approach continuously while fostering an environment where everyone feels invested in achieving high-quality outcomes through effective communication around AQL levels.
How China Inspection Pro Excels in Documentation
China Inspection Pro stands out among its peers by prioritizing exceptional documentation practices within its QA services. Their commitment to defining quality is evident through their meticulous record-keeping systems that ensure every aspect of their QC processes is documented comprehensively—from initial inspections using AQL samples right through to final reporting stages based on ISO standards compliance.
Moreover, China Inspection Pro employs advanced technology solutions designed specifically for managing documents related to quality inspection activities efficiently—a feature that many other quality control companies may overlook or underutilize! This innovative approach not only enhances accuracy but also streamlines workflows so teams can focus on delivering top-notch results rather than getting bogged down by administrative tasks.
In summary, when it comes down to effective documentation within QA frameworks like those employed by China Inspection Pro—their ability not only improves operational efficiency but also reinforces their reputation as leaders within the industry while helping clients achieve unparalleled success through rigorous adherence to best practices surrounding AQL samples.
Overlooking the Role of Training

In the world of quality assurance, overlooking the role of training can lead to a cascade of errors that compromise product integrity. Quality control is not just about following protocols; it’s about understanding them deeply to ensure effective quality inspection at every stage. When staff are inadequately trained, even the best-defined quality standards can fall flat, leading to misinterpretations and costly mistakes in AQL samples.
Training Staff on Quality Control Fundamentals
Training staff on the fundamentals of quality control is essential for establishing a robust QC quality control process. Employees need to be well-versed in what it means to define quality and how it aligns with various ISO standards relevant to their industry. By equipping team members with a solid foundation in these principles, organizations can significantly enhance their ability to conduct accurate inspections and effectively interpret AQL levels.
Moreover, ongoing training sessions should focus on real-world applications of these concepts, including hands-on practice with AQL samples and scenarios they may encounter during inspections. This practical approach ensures that employees are not just passive recipients of information but active participants in the learning process. Ultimately, well-trained staff become valuable assets who contribute meaningfully to maintaining high-quality standards.
Continuous Improvement through Education
Quality assurance is an ever-evolving field where continuous improvement through education is paramount. Regularly updating training programs ensures that employees remain informed about new ISO standards and emerging best practices in quality control companies worldwide. This commitment to education fosters a culture of excellence where team members feel empowered to seek knowledge and apply it effectively.
Furthermore, investing in advanced training opportunities—such as workshops or certifications—can lead to significant improvements in how teams handle AQL samples during inspections. When staff are educated about statistical sampling techniques and their relevance in QC processes, they become adept at making informed decisions that enhance overall product quality. Continuous improvement isn't just a buzzword; it's a strategic approach that pays dividends over time.
Leveraging Knowledge for Better AQL Samples
Leveraging knowledge gained from comprehensive training can dramatically improve the handling of AQL samples throughout the inspection process. Organizations that prioritize education create an environment where employees are encouraged to share insights and strategies for optimizing quality control measures effectively. This collaborative mindset leads to innovative solutions for common challenges faced during inspections.
Additionally, when teams understand how different ISO standards apply specifically to their work context, they can better tailor their approaches for more accurate results during quality inspection processes. The synergy between knowledgeable staff and effective procedures creates a cycle of continuous improvement that enhances product integrity over time while minimizing errors related to misinterpreted AQL levels or inconsistent QC practices. In essence, investing in employee education isn't just beneficial; it's essential for achieving excellence in quality assurance.
Conclusion
In wrapping up our exploration of quality control and assurance, it’s clear that understanding AQL samples is crucial for maintaining high standards. The common pitfalls in quality inspection can be avoided by adhering to best practices and fostering a culture of continuous improvement. By focusing on defining quality through established ISO standards and leveraging the expertise of quality control companies, businesses can significantly enhance their QC processes.
Key Takeaways from Quality Control Insights
One major takeaway is the importance of accurately interpreting AQL levels to prevent missteps that could compromise product integrity. Consistency in QC quality control procedures not only ensures reliable outcomes but also builds trust with clients and stakeholders alike. Additionally, effective training and documentation serve as the backbone for successful quality assurance initiatives, making it essential to prioritize these areas.
Enhancing Quality Assurance Through Best Practices
To enhance your approach to quality assurance, consider implementing statistical sampling techniques when dealing with AQL samples; this will provide a more robust framework for decision-making during inspections. Establishing clear guidelines based on ISO standards can streamline processes while ensuring compliance across all levels of production. Moreover, regular audits and updates to your QC protocols will help maintain consistency and adapt to evolving industry requirements.
Next Steps for Improving Your Quality Processes
Moving forward, take actionable steps by reviewing your current QC practices against established benchmarks in the industry; this includes assessing how well you define quality within your organization. Engage with reputable quality control companies to gain insights into innovative methods for improving your inspection processes. Finally, invest in ongoing training programs that empower staff with the knowledge necessary for optimizing AQL samples and achieving excellence in every batch produced.
